|
| FDA Approves
Mexoryl |
|||||
 |
|
||||
|
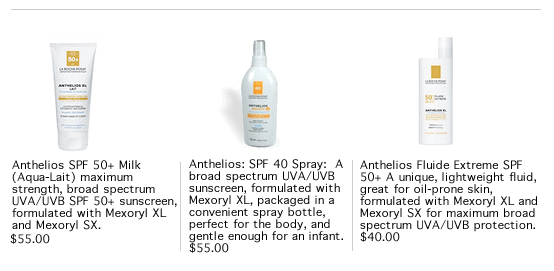
Today the FDA announced the long awaited approval of the sunscreen ingredient Mexoryl. Mexoryl has been universally available in Europe and Canada for years but has been the hard-to-find sunscreen in America. Mexoryl is presently found in La Roche-Posay's Anthelios sun care line. Classically, the SPF labeling on a sunscreen has referred to the blocking of UVB radiation. Though we know that UVB is the major culprit in sunburns and non-melanoma skin cancer, UVA has been shown to also play a role. UVA significantly affects the aging of the skin, causing loss of elasticity and wrinkles. Its role in skin cancer has also been considered. Though there are a few UVA blockers already on the market, there has been concern that they have not been as stable as one might prefer. Anthelios with Mexoryl has been shown to be superior by its users. SkinCareLab founder, Dr Brad Katchen is excited about Mexoryl coming to the States, " I have always suggested Anthelios to my patients who are most at risk for skin cancer and also those who have medical conditions that are affected by sunlight. Now with the FDA approval it will be easier to obtain. Hopefully more people will use it religiously and be protected from the harmful effects of the sun.” For those who will be enjoying the summer don’t forget your Anthelios sunscreen. Perhaps equally important, remember to reapply your sunscreen every two hours. Also, try to wear protective clothing and avoid the sun during the peak UV hours hours of 10 am-2 pm For those who will be taking a break from the beach and spending the summer in the city, SkinCareLab would like to offer you a special. Book yourself a refreshing body treatment, whether a GrapeSeed Scrub, Papaya Pineapple Scrub, or a Get-Away Glow body polish, and receive a complementary pedicure. Click on the coupon below for more information. SkinCareLab wishes you a safe summer season!!
|
|||||

|
|||||

|
|
|
|||
Try Some Great Anthelios Sun Care At SkinCareLab.com